Vaccine ingredients and production in other nations are substantially the same. Due to the lower effectiveness of the inactivated flu vaccine in older people a vaccine containing an adjuvant was introduced for the 2018-19 season.
Linking to a non-federal website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
Flu vaccine ingredients 2018. This years seasonal flu vaccine contains protection against 4 strains of flu virus. All adult flu vaccines are given by injection into the muscle of the upper arm. All other ingredients weigh a few milligrams thousandths of a gram or even less.
Recommended composition of influenza virus vaccines for use in the 2017-2018 northern hemisphere influenza season - full report pdf 117kb. Also listed are substances used in the manufacturing process. Td Tenivac 112019 aluminum phosphate formaldehyde sodium chloride water Td TDVAX 92018 aluminum phosp hate formaldehyde thimerosal.
An AMichigan452015 H1N1 pdm09-like virus an. The committee recommended that the trivalent formulation influenza vaccines for the US. Starting with the 2018-2019 influenza season most of the regular-dose egg-based flu shots and all of the recombinant and cell-grown flu vaccines in the United States are quadrivalent.
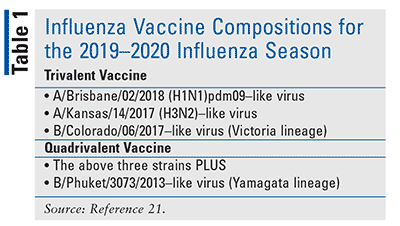
Flu vaccines are very safe. 2018-2019 influenza season contain the following. Vaccine recipients and guardians should be instructed that annual vaccination is recommended.
Links with this icon indicate that you are leaving the CDC website. The ingredient called adjuvant MF59 is a natural compound that can be made from vegetable oils or more commonly shark liver oil. It is recommended that quadrivalent vaccines containing two influenza B viruses contain the above three viruses and a BPhuket30732013-like virus.
An AGuangdong-MaonanSWL15362019 H1N1pdm09-like virus. Most injected vaccines contain 05 millilitres of liquid in other words a few drops. The four strains are.
Flu season Skip directly to site content Skip directly to page options Skip directly to A-Z link Centers for Disease Control and Prevention. Having the flu vaccine will also stop you spreading flu to other people who may be more at risk of serious problems from flu. This is a substance that strengthens and lengthens the immune response to the vaccine and resulted in better prevention of flu in people aged 65 or over in 2018-19 and 2019-20 seasons.
Vaccine Excipients Adenovirus vaccine. How effective is the 2018 flu shot. This list refers to the type 4 and type 7 adenovirus vaccine tablets licensed in the US.
Here are some ingredients youll find in the flu shot. It is recommended that quadrivalent vaccines for use in the 2018-2019 northern hemisphere influenza season contain the following. An AMichigan452015 H1N1pdm09-like virus.
Flu vaccine side effects. Many flu vaccines are made by growing the viruses inside fertilized chicken eggs. Apart from this the main ingredient in vaccines is water.
HEPES 2 human serum albumin 05 - 07 sodium chloride USP 5 Mannitol USP neomycin polymyxin B 50 Glycerin USP 025 phenol USP. The purpose of each ingredient is either to make the vaccine effective or ensure that it is safe. These are recommended by the World Health Organization WHO as the strains most likely to be circulating this season.
The Centers for Disease Control and Prevention CDC cannot attest to the accuracy of a non-federal website. Following a meeting in Geneva this week the World Health Organizations WHOs flu vaccine advisory group today recommended changing two of the four components for quadrivalent vaccines to be produced for the Northern Hemisphere 2018-19 flu season. 1988 influenza vaccine the decision.
For further information on individual vaccines please refer to the relevant Product Information document or Consumer Medicine Information document. In the 20192020 influenza season all regular-dose flu shots and all recombinant influenza vaccine in the United States are quadrivalent. Influenza vaccine effectivness study for the 2018-2019 US.
2017-2018 Formula If Guillain Initial US Approval. For further information about the use of seasonal influenza vaccines in children please refer to the TGA web statement. 2018 seasonal influenza vaccines for use in children.
Many vaccines for the flu and other viral infections contain similar ingredients. The flu vaccine is recommended as the best way to protect against the flu but its no silver bullet. Recommended composition of influenza virus vaccines for use in the 2018-2019 northern hemisphere influenza season 22 February 2018.
The key ingredient in all vaccines is one or more active ingredients see below. This new vaccine contains an extra ingredient called an adjuvant that promotes a stronger immune response to the flu vaccine. It can take 10 to 14 days for the flu vaccine to work.
Cari Blog Ini
Label
- 1000rr
- 1040ez
- 1080p
- 12th
- 15th
- 1600x900
- 1920x1080
- 1969
- 1971
- 2000
- 2007
- 2013
- 2015
- 2016
- 2017
- 2018
- 2019
- 2020
- 2021
- 2160p
- 2560x1600
- 2pac
- 38mm
- 4matic
- 50th
- 60th
- a380
- above
- abscess
- abstract
- academia
- accent
- accord
- accounting
- acorn
- acoustic
- acrylic
- activate
- activity
- actor
- actress
- acts
- adaan
- adapter
- adding
- adelaide
- adidas
- aditya
- adjust
- adjustable
- administration
- adopt
- adrenalin
- adults
- advantages
- advice
- aerial
- aesthetic
- african
- after
- against
- agency
- ages
- agrawal
- agreement
- airbus
- akira
- album
- alcohol
- alexa
- alien
- allocation
- alphabet
- alternative
- alzheimers
- amazing
- amazon
- amazonca
- america
- american
- anak
- android
- aneka
- angebote
- angles
- angry
- animal
- animals
- animated
- Animation
- anime
- anna
- anne
- annie
- anniversary
- answers
- anti
- appetit
- apple
- application
- apply
- apron
- arabic
- architectural
- area
- ariel
- army
- around
- artists
- artwork
- asam
- asem
- asian
- asli
- astigmatism
- astrazeneca
- attorney
- audio
- austin
- australia
- authentic
- auto
- autumn
- availability
- avatar
- avengers
- average
- ayam
- azerbaijan
- baba
- babi
- babies
- baby
- back
- background
- backgrounds
- backpack
- backyard
- badan
- badge
- bahasa
- bajaj
- bakar
- baking
- bakpao
- bakso
- balaji
- balance
- balap
- balcony
- bali
- ball
- balloon
- balls
- band
- bandeng
- bands
- bandung
- bank
- banyak
- barbeque
- barn
- basah
- baseboards
- based
- baso
- bass
- bathroom
- bathtub
- batman
- bawang
- bayam
- beach
- bean
- bear
- beatrice
- beautiful
- beauty
- bebek
- become
- bedroom
- bedrooms
- beginner
- beginners
- beginning
- bein
- bekal
- believe
- belle
- belut
- bembeng
- bening
- beras
- berkuah
- best
- betutu
- bexsero
- bharma
- bible
- bicycle
- bihun
- bikang
- bike
- bikes
- bikin
- bill
- biohazard
- bird
- birth
- birthday
- birus
- bistik
- bites
- black
- blank
- blanket
- blaziken
- bleed
- blends
- blessed
- blick
- blissey
- block
- blossom
- blue
- bluetooth
- blush
- bobbin
- bogor
- bohemian
- boho
- bola
- bolen
- bollywood
- bolot
- bolu
- bomb
- book
- bookey
- books
- boost
- border
- boston
- botafogo
- botox
- bottle
- boujee
- bouquet
- bowood
- boxwood
- boyfriend
- boys
- bpjs
- brand
- breaking
- breast
- brick
- bridal
- bride
- bridge
- brock
- brooklyn
- brother
- brown
- brownies
- brush
- buah
- buat
- bubbles
- bubur
- buddha
- budget
- budgeting
- bugis
- bugs
- buka
- bull
- bulldog
- bumbu
- bundle
- bungalow
- buono
- burton
- business
- butter
- butterfly
- buying
- cajun
- cake
- cakes
- calculate
- calendar
- call
- calla
- calligraphy
- calories
- camaro
- camera
- cameras
- camo
- campur
- canada
- cancelled
- candy
- canvas
- capcay
- cape
- captions
- cara
- card
- cardinal
- cards
- cargo
- carnivores
- carolina
- cars
- cartier
- case
- casetify
- cast
- castle
- cats
- caulk
- causes
- cave
- cbr1000rr
- cecek
- cell
- cemilan
- cendol
- centerpiece
- centerpieces
- centre
- ceramic
- certificate
- chair
- chakra
- champion
- change
- chaplin
- chapstick
- character
- characters
- charge
- charizard
- charles
- charlie
- charmin
- chart
- charts
- chauth
- cheap
- checklist
- cheerleading
- chek
- chennai
- cherry
- chicago
- chicken
- chiffon
- child
- children
- childrens
- chinese
- chocolatos
- choirudin
- chore
- christmas
- chudi
- cider
- cigar
- cilok
- cilor
- cimol
- circle
- city
- cityscape
- civic
- clancys
- claro
- class
- clean
- cleaning
- clemente
- cling
- clinic
- clip
- clipart
- clothes
- clothing
- clover
- club
- coastal
- coat
- coco
- code
- coffee
- coffin
- coins
- coklat
- collage
- colonial
- color
- colorado
- colored
- colorful
- coloring
- colors
- colour
- colts
- columbus
- comfort
- command
- commentators
- commercial
- common
- compact
- companies
- comparing
- complete
- completely
- components
- comprehenshion
- comprehension
- computer
- computers
- concept
- conjugate
- connect
- consent
- conte
- contemporary
- contoh
- contraindications
- control
- controller
- controllers
- conventions
- cook
- cookies
- cool
- copier
- core
- corelle
- corner
- cornflakes
- corona
- coronavirus
- corps
- correct
- correcting
- corvette
- cost
- cough
- country
- couple
- coupon
- couponing
- coupons
- courthouse
- courtyard
- cover
- covered
- covid
- crafts
- crane
- cream
- creamer
- creases
- create
- createshake
- creative
- crispy
- crocodile
- croissant
- cross
- crossing
- crossword
- crunch
- crying
- crystal
- cuba
- cucur
- cudi
- cumi
- cups
- currency
- curry
- cursive
- custom
- customizable
- customized
- cute
- cyber
- cyberpunk
- cycle
- dadar
- daffodil
- daging
- daily
- dalgona
- dallas
- dame
- dance
- dancing
- dark
- dashboard
- date
- daughter
- davidson
- dawn
- deals
- debate
- debby
- debm
- decals
- decor
- decorating
- decoration
- decorations
- defender
- define
- definition
- delete
- deleted
- dello
- delonghi
- deluge
- denominators
- dental
- desert
- design
- designer
- designs
- desk
- desktop
- desktops
- dessert
- destiny
- devi
- device
- diabetic
- diagnosed
- diana
- dicembre
- different
- digest
- digit
- digraphs
- dijual
- dikhaiye
- dimensional
- dimsum
- dinamica
- dinosaur
- direct
- disc
- discord
- disease
- diseases
- disney
- disneyland
- display
- distemper
- distribution
- ditto
- division
- djum
- dnealian
- doberman
- doctor
- doctors
- documents
- does
- dogs
- dolch
- doll
- dollar
- dolphin
- domestic
- donat
- donuts
- door
- dora
- dorayaki
- dormify
- dosage
- dose
- dots
- down
- download
- downloadables
- downloader
- dragon
- dragonfly
- draw
- drawing
- dreams
- dress
- dresses
- driftwood
- drip
- drive
- drivers
- drone
- dtap
- dude
- duke
- dulha
- dulhan
- dunkin
- durga
- during
- dusanovac
- dynamite
- eagle
- earbuds
- earphones
- ears
- easy
- ebola
- economics
- edge
- edging
- education
- eevee
- effect
- effectiveness
- effects
- eggs
- eiffel
- elastique
- electricity
- elements
- eleven
- elizabeth
- elsa
- elves
- embossed
- embroidered
- employee
- empuk
- enak
- enchanted
- engine
- english
- enter
- enterprise
- entry
- envelope
- equations
- equivalent
- ergonomic
- escort
- espresso
- essential
- ethan
- etsy
- evaluation
- event
- everyone
- eviction
- evolution
- evolutions
- excel
- excelworksheetxlworkbookworksheetsget_item1
- exercice
- exercises
- explained
- explosion
- extensions
- extra
- eyebrows
- eyelash
- fabric
- face
- faces
- fact
- facts
- fall
- Family
- famous
- fancy
- Fantasy
- farm
- farmhouse
- fastest
- fauci
- feather
- feature
- feline
- felt
- female
- fence
- ferrari
- fessier
- fever
- fiction
- field
- fight
- figures
- file
- files
- fill
- financial
- find
- fingers
- fire
- firefighter
- firefly
- fires
- first
- firstever
- fish
- fitness
- five
- fixed
- flag
- flash
- flask
- flat
- fletcher
- flip
- floral
- florida
- flower
- flowers
- flue
- fluffy
- font
- food
- footage
- football
- ford
- foremost
- forest
- form
- forms
- formula
- formulas
- fortnite
- foto
- found
- fountain
- four
- fractions
- framed
- frames
- franxx
- free
- french
- friday
- friend
- frog
- from
- front
- frozen
- fruit
- full
- funding
- funky
- funny
- fvrcp
- gabus
- gacoan
- gadgets
- gagal
- galaxy
- gallery
- gambarnya
- game
- games
- gamestop
- gaming
- ganesh
- gardasil
- garden
- garland
- garpu
- gastly
- gaun
- gazzetta
- gears
- geico
- gender
- generalization
- generator
- geneva
- gens
- geometric
- gepuk
- gerem
- germania
- germany
- ghin
- giada
- giants
- gift
- gifts
- gilimanuk
- giovanna
- girls
- girly
- given
- glacier
- glass
- glazed
- glitch
- glizzy
- glock
- glow
- glycol
- gnome
- goddess
- godog
- gold
- gomez
- good
- goreng
- gorengan
- göttingen
- gown
- gowns
- goyang
- grade
- graders
- gradient
- grading
- graffiti
- grammar
- gran
- granada
- grande
- graph
- graphic
- graphics
- gratis
- grave
- gray
- great
- green
- grey
- grid
- grinch
- grinder
- groom
- grounds
- group
- gudangan
- gudeg
- guidebook
- guitar
- gula
- gulai
- gulf
- gulung
- gums
- gurame
- guys
- hack
- hackney
- hahnemuhle
- hair
- half
- halloween
- hallway
- hand
- handheld
- hands
- handwriting
- hanging
- hanuman
- happy
- hard
- hardware
- harley
- hartford
- harvest
- hatch
- hatchback
- have
- hd7447
- head
- headboard
- header
- headliner
- headlines
- headphones
- hear
- heel
- hepatitis
- herb
- heroine
- herringbone
- hidden
- hiden
- high
- highland
- hiroin
- hiscox
- History
- hitam
- hobby
- hogwarts
- holder
- hollywood
- home
- homemade
- homeowners
- homeschool
- homework
- honda
- honeycomb
- hoothoot
- horizontal
- horse
- hotel
- hotwire
- house
- housewarming
- houzz
- hulu
- hummingbird
- hunkwe
- hunt
- hunting
- huracan
- husband
- hustle
- hybrid
- iced
- icing
- iconic
- ideas
- ikan
- ikea
- ikonick
- illusion
- image
- imagebank
- images
- implants
- impressions
- indian
- indianapolis
- indications
- indigo
- indomie
- indonesia
- indonesianya
- industrial
- infinity
- influenza
- information
- ingredients
- initial
- injections
- injury
- inkblot
- insert
- inspiration
- inspirational
- install
- insurance
- interior
- intermediate
- internasional
- interview
- intra
- introduced
- invented
- inventor
- invitation
- invoice
- ipad
- iphone
- iran
- ireland
- iron
- islamic
- italian
- item
- jack
- jadi
- jadul
- jagung
- jahe
- jakarta
- january
- japanese
- jazz
- jeep
- jemblem
- jenang
- jengkol
- jenner
- jepang
- jesus
- jets
- jmsb
- jobs
- jogja
- john
- johnny
- johnson
- join
- jojo
- jolteon
- jordan
- journal
- jualan
- just
- kacang
- kaiser
- kalio
- kambing
- kannada
- kapal
- kare
- kari
- karstadt
- kate
- katiri
- katsu
- kaveri
- kawasaki
- keatas
- kebuli
- kecap
- keju
- kekinian
- kelly
- kemangi
- kembang
- kembung
- kemplang
- kentang
- kentucky
- kepala
- kepiting
- kepok
- kerala
- kering
- keripik
- ketan
- keurig
- keys
- khas
- kids
- kindergarten
- kindle
- kindness
- king
- kinkade
- kiss
- kitchen
- kits
- kitten
- klasik
- knot
- koala
- kobe
- kodak
- kodi
- kohls
- konidela
- konro
- kopi
- korea
- korean
- krecek
- krepes
- krishna
- krispi
- kristen
- kuah
- kukus
- kulit
- kuning
- kuniran
- kuno
- kylie
- label
- labels
- lacrosse
- ladies
- ladybug
- lake
- lakshmi
- lalapan
- lamborghini
- land
- landscape
- landscapes
- lapis
- laptop
- large
- lasagna
- laser
- latest
- latin
- latte
- lawsuit
- laxmi
- leaf
- league
- learning
- lease
- leather
- leave
- left
- legendary
- lehenga
- lembut
- lens
- leopard
- lesson
- lets
- letter
- letters
- level
- lezat
- liability
- library
- licence
- license
- life
- lifestyle
- light
- lighter
- lighting
- lights
- like
- lilin
- lilit
- lilly
- lily
- limited
- line
- list
- lists
- little
- live
- living
- lizzo
- lobby
- lock
- lodeh
- login
- logo
- lombok
- london
- long
- lontong
- look
- loose
- lord
- lose
- louis
- love
- lovers
- lowes
- lumer
- lumpia
- lumpur
- luther
- luxury
- lyme
- lyric
- lyrics
- machine
- mackinac
- macrame
- madden
- madison
- madura
- magicom
- magnet
- magnetic
- mahal
- mahalakshmi
- mahalaxmi
- maintenance
- majesty
- makanan
- make
- maker
- manchester
- manga
- manis
- mantel
- manten
- manual
- manufactures
- manufacturing
- many
- marble
- marilyn
- markers
- market
- marketplace
- marks
- marmer
- marriage
- martabak
- maryland
- masak
- masakan
- master
- mata
- matah
- math
- matted
- maury
- mayonaise
- mcdonalds
- mcgra
- meaning
- measles
- measure
- meat
- medan
- medicaid
- medicare
- meeting
- mehandi
- mehndi
- mein
- membrane
- membuat
- meme
- memes
- meningitis
- meningococcal
- mens
- mentega
- menu
- merah
- mercedes
- merck
- mercon
- mermaid
- merry
- messages
- metal
- mexican
- michael
- mickey
- micrometer
- microwavable
- migrate
- mikasa
- miles
- military
- miller
- milor
- minecraft
- mini
- miniature
- minimalist
- minimized
- minuman
- minus
- minyak
- mirage
- mirror
- miso
- misoa
- missing
- mission
- mizuno
- mobile
- mobiles
- mockup
- modern
- molen
- moltres
- monday
- mondrian
- monet
- money
- monitor
- monitors
- monroe
- monster
- month
- monthly
- moon
- morris
- mosaic
- moth
- mother
- mothers
- motivation
- motivational
- motor
- mountain
- mountains
- mouse
- mouth
- move
- moves
- movie
- movies
- moving
- mrna
- much
- mugs
- multi
- multiple
- multiplication
- mural
- murals
- Music
- Musical
- muslim
- mustang
- myford
- Mystery
- nagasari
- nail
- name
- names
- nanas
- nantucket
- narayan
- narrow
- nasa
- nascar
- nashville
- nasi
- nastar
- national
- native
- nativity
- natural
- naturally
- nature
- navratri
- navy
- near
- necklace
- need
- nelayan
- nencini
- neopar
- nescafe
- netgear
- network
- neuropathy
- neutral
- newborn
- News
- newton
- nezuko
- night
- niharika
- ninja
- nintendo
- nissan
- noise
- nordstrom
- north
- northern
- norway
- note
- notes
- notice
- notre
- nouns
- novel
- nożna
- number
- nurse
- nursery
- nutrition
- nyonya
- observation
- observations
- ocean
- office
- official
- often
- ohio
- olahan
- omega
- omnivores
- oncom
- online
- open
- opens
- opinion
- orange
- orchid
- order
- oreo
- organisms
- original
- orlando
- ornaments
- oseng
- outdoor
- outlet
- outline
- outlook
- outside
- oval
- over
- overwatch
- oxford
- packers
- padang
- page
- pages
- paint
- paintable
- painted
- painting
- paintings
- pair
- pais
- palembang
- palette
- pallets
- pancho
- panda
- pandan
- panel
- panera
- panggang
- panis
- panoramic
- pans
- panther
- paper
- paranoid
- paris
- parivar
- parks
- paru
- parvati
- parvo
- pass
- password
- pastel
- pasteur
- pastry
- patches
- patent
- path
- pattinson
- payment
- peaceful
- peach
- peacock
- pearls
- pearson
- pectoraux
- pediatricians
- peel
- peerless
- pencil
- pencils
- penius
- pentol
- penulisan
- penurun
- people
- percentage
- perennial
- periodic
- permanent
- permanente
- personalized
- pertussis
- pesmol
- pewter
- pexel
- pfizer
- philippines
- philips
- philly
- phone
- phones
- photo
- photography
- photos
- photoshoot
- picher
- pick
- picks
- pics
- picture
- pictures
- pieced
- pier
- pigs
- piłka
- pillow
- pimpernel
- piñata
- pindang
- pineapple
- ping
- pink
- pintu
- pipe
- pirate
- pisang
- pitbull
- pittsburgh
- pixel
- pixelmon
- place
- plague
- plaid
- plan
- planeta
- planner
- planning
- plans
- plant
- planter
- plants
- plaster
- play
- playstation
- plot
- plots
- plus
- plush
- pneumococcal
- pneumonia
- podcast
- poem
- pokemon
- pokémon
- polio
- polos
- polyethylene
- pomeranian
- pool
- porch
- positive
- poster
- posters
- postmates
- powder
- power
- practice
- praktis
- prayer
- predator
- pregnant
- premier
- prerunner
- preschool
- prescription
- present
- presto
- pretty
- preventable
- prezzo
- price
- primarina
- princess
- principles
- printable
- printables
- printer
- printing
- prints
- private
- probabili
- problems
- process
- program
- proof
- propagate
- proposal
- pros
- protection
- publish
- puding
- puff
- pukis
- pulsar
- pump
- pumpkin
- pumps
- punctuation
- puppy
- purex
- purge
- purple
- pusheen
- putih
- puyuh
- puzzle
- puzzles
- python
- quality
- quartz
- queen
- quest
- questions
- quidditch
- quilt
- quilted
- quiz
- quote
- quotes
- quran
- rabbit
- rabies
- racing
- rack
- radha
- radhe
- rahasia
- rain
- rainbow
- rainbows
- raised
- rapper
- rarest
- rash
- rate
- rating
- rawon
- razer
- reaction
- readiness
- reading
- ready
- real
- reality
- rebel
- rebus
- receive
- reception
- recife
- recipe
- recommendations
- reconstruction
- record
- rectangle
- rectangular
- redflagdeals
- reds
- reducing
- reference
- registry
- regrouping
- relax
- release
- religious
- removable
- remove
- rendang
- rent
- renyah
- repeating
- replace
- replacement
- repsol
- republic
- research
- resep
- residential
- resolution
- respiration
- respond
- response
- rest
- restaurant
- reteaching
- retrieve
- retro
- review
- reviews
- rewards
- ribbon
- rice
- richard
- ridge
- ring
- rings
- risol
- risoles
- ritter
- robert
- roblox
- rock
- rocket
- rocks
- roll
- romantic
- room
- rose
- roses
- ross
- roti
- round
- rover
- royalty
- royco
- rubicon
- ruby
- rudy
- rugby
- rujak
- rule
- ruler
- rumahan
- ruptured
- russian
- rustic
- ryan
- safety
- sakura
- sala
- salary
- sale
- salmon
- sambal
- samsung
- sand
- sandlot
- sanofi
- santa
- santan
- santas
- saphire
- sapi
- sarabba
- sarden
- sarnia
- sate
- saus
- sauth
- sawi
- saxon
- sayur
- scarf
- scenery
- scenes
- schedule
- school
- sciatica
- science
- sconto
- score
- scotland
- scott
- scrabble
- screen
- screensavers
- sculpture
- seashell
- seaside
- season
- seats
- seattle
- seblak
- second
- section
- sederhana
- sedona
- sego
- selke
- semur
- sendok
- seniors
- senses
- sentence
- sentences
- septic
- serani
- serenity
- series
- serundeng
- server
- services
- sets
- shapes
- shared
- shark
- shed
- sheet
- shein
- shelby
- sherwin
- shingle
- shingles
- shingrix
- shiny
- shipping
- shirdi
- shirt
- shirts
- shiv
- shiva
- shoe
- shoes
- shop
- shopping
- shops
- Short
- shorten
- shot
- shots
- shouldn
- show
- shree
- shri
- side
- siege
- sign
- signature
- signs
- sikhane
- silent
- simple
- since
- singapore
- singkong
- sinjay
- sinopsis
- siser
- sister
- site
- sites
- siwa
- size
- sizes
- skate
- skateboard
- sketchup
- skiing
- skills
- skull
- skyline
- slat
- slime
- slip
- slonem
- slow
- slowpoke
- small
- smallpox
- smart
- smoke
- snake
- snap
- snapchat
- sneakers
- snoopy
- snowman
- soak
- soccer
- social
- society
- soft
- solo
- someone
- song
- songs
- sony
- sore
- sorts
- sosis
- soto
- sound
- soundtrack
- soup
- south
- southwestern
- space
- spade
- spangled
- spanish
- spark
- speaker
- speakers
- spelling
- spider
- spiderman
- spine
- splash
- splatter
- split
- spoilers
- spoofing
- Sport
- sportback
- sports
- spray
- spring
- squad
- square
- squishmallows
- srbija
- stadium
- staedtler
- stainless
- stains
- stand
- standing
- star
- starbucks
- starcraft
- stark
- starry
- start
- starters
- state
- states
- status
- steam
- steelers
- stem
- stencil
- step
- stephen
- stewart
- stick
- stickers
- stik
- stock
- stone
- stonks
- stool
- stop
- stories
- story
- strawberry
- stream
- streaming
- street
- strikers
- students
- stuffed
- stup
- styles
- submarine
- subtraction
- success
- succulent
- sudoku
- sugar
- sumatra
- sumif
- summer
- sumsum
- sunday
- super
- supercar
- superman
- superteacher
- supplies
- supply
- supreme
- surabaya
- susu
- suzani
- swab
- switch
- swollen
- sword
- symptoms
- synthesis
- system
- table
- tables
- tablet
- tacoma
- tahu
- tahun
- taiga
- takaran
- take
- takoyaki
- taliwang
- tamales
- tamil
- tangled
- tanpa
- tape
- tapestry
- target
- targets
- tarot
- tattoo
- taunt
- tawar
- tdap
- teach
- teacher
- teachers
- teaching
- teal
- teams
- tech
- technology
- teddy
- teens
- telugu
- telur
- tempe
- template
- templates
- temporary
- tennessee
- tennis
- tens
- tent
- tepung
- terasi
- terbaru
- teriyaki
- terms
- terong
- test
- testing
- testosterone
- tetelan
- texas
- text
- textured
- thanksgiving
- that
- theme
- themed
- thermasol
- thermos
- this
- thomas
- thread
- three
- thrones
- throttle
- thursday
- tiffany
- tiger
- tiktok
- tile
- tiles
- time
- timelapse
- timeline
- tint
- tiram
- tires
- tirupati
- titan
- today
- toddler
- toilet
- tomat
- tomorrow
- toner
- tongkol
- tony
- tool
- toppers
- torrent
- touch
- toxic
- toyota
- toys
- trac
- track
- tracking
- tractor
- trading
- traditional
- train
- training
- transparan
- transparent
- travel
- travis
- treatment
- tree
- trellis
- trend
- trim
- trippy
- trivia
- tropical
- trout
- truck
- true
- tucson
- tumis
- tumpeng
- tundra
- turkey
- turkeys
- turn
- turtle
- tusuk
- tutorial
- twinrix
- twins
- type
- typhoid
- udang
- ukuran
- ultra
- umbreon
- under
- undercooked
- underwear
- unemployment
- ungkep
- unicorn
- unique
- unit
- united
- university
- unlicensed
- unlike
- untuk
- update
- upload
- uptodown
- urdu
- urine
- usus
- vaccinated
- vaccinating
- vaccination
- vaccinations
- vaccine
- vaccines
- vacuum
- valentine
- valhalla
- value
- varicella
- vector
- vegas
- vegetable
- vehicle
- venetian
- venue
- verbs
- verse
- versions
- versus
- vertical
- vibia
- vibrant
- video
- videos
- views
- vinci
- vine
- vineyard
- vintage
- vinyl
- virus
- visualisation
- vivo
- vocabulary
- volkswagen
- vrijeme
- vuitton
- waffle
- walk
- walkthrough
- wall
- wallpaper
- wallpapers
- walls
- walmart
- waluh
- warfare
- warm
- watch
- watches
- water
- watercolor
- watercolors
- wave
- wayfair
- weakness
- website
- websites
- wedding
- wednesday
- week
- weekly
- weight
- weighted
- west
- Western
- westie
- what
- wheelchair
- when
- where
- while
- whimsical
- white
- whitetail
- whole
- whooping
- widget
- wife
- wifi
- wiki
- wild
- window
- windows
- wine
- winelands
- winsor
- winter
- wire
- wireless
- wishes
- wisman
- with
- wolf
- woman
- womens
- wonder
- wonderful
- wood
- wooden
- woodland
- word
- words
- work
- workers
- working
- workplace
- works
- worksheet
- worksheets
- worksheetsorg
- world
- woven
- wristbands
- writing
- xray
- xvideos
- yakiniku
- yang
- yantra
- year
- yeezy
- yeezys
- yellow
- yoda
- york
- your
- youtube
- zebra
- zedge
- zelda
- zero
- zoom
- zora
- żywo

